Abstract
Purpose Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) with a heterogeneous clinical course. Allogeneic hematopoietic cell transplantation remains the only curative therapy, but its significant morbidity and mortality require careful candidate selection. Therefore, accurate disease risk prognostication is critical for treatment decision making.
Patient and Methods Registry data were retrieved from patients diagnosed with MF between January 2000 and October 2021 in 59 Spanish institutions. A total of 1,386 patients were randomly divided into a training set (80% of the cohort) and a test set (20% of the cohort). A machine learning (ML) technique (random forests) was used to model overall survival in the training set and validate the results in the test set.
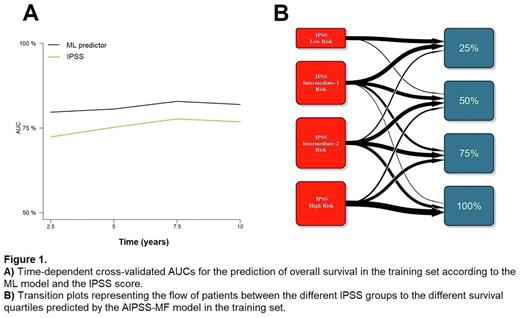
Results The ML model, based on eight clinical variables determined at diagnosis, achieved high accuracy in predicting overall survival (training set c-index, 0.750; test set c-index, 0.744) and leukemia-free survival (training set c-index, 0.697; test set c-index, 0.703). We confirmed the superiority of the model compared with the IPSS in the prediction of survival at all time points evaluated (Figure 1A). Importantly, the model was superior to the IPSS regardless of age range (<60 or >60 years). We derived 4 equally distributed groups of patients from the model predictions, comprising 25% of patients each. By comparing this distribution with the IPSS risk groups, we observed that circa 50% of patients were reassigned to a different risk group by the ML algorithm (Figure 1B). Additionally, we did not observe any benefit from including MPN driver mutations or high-risk somatic mutations in the model. Furthermore, the ML model outperformed the prognostic accuracy of the MIPSS70 in primary MF and the MYSEC-PM in secondary MF.
Conclusion The AIPSS-MF (Artificial Intelligence Prognostic Scoring System for Myelofibrosis) is based exclusively on clinical variables that are readily available in any healthcare facility. This simple model has demonstrated high prognostic accuracy for predicting survival in patients with primary and secondary MF, outperforming other well-established risk scoring systems. An interactive web calculator of this model can be accessed in the following link: https://geneticsoncohematology.com/MF/
Disclosures
Mosquera Orgueira:Roche, Amgen, AstraZeneca, Janssen, Abbvie, Takeda, Brystol, GSK, Pfizer,: Consultancy, Honoraria, Research Funding. Ferrer Marin:Incyte: Research Funding. González de Villambrosia:Incyte: Honoraria; Janssen: Honoraria; Takeda: Honoraria; EusaPharma: Honoraria. Gómez-Casares:Novartis: Speakers Bureau; Pfizer: Speakers Bureau; BMS: Speakers Bureau. Gasior Kabat:Brystol Myers Squibb: Other: Advisory Board ; Eusa Pharma: Speakers Bureau; Novartis: Other: Advisory Board , Speakers Bureau. Hernández Rivas:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal